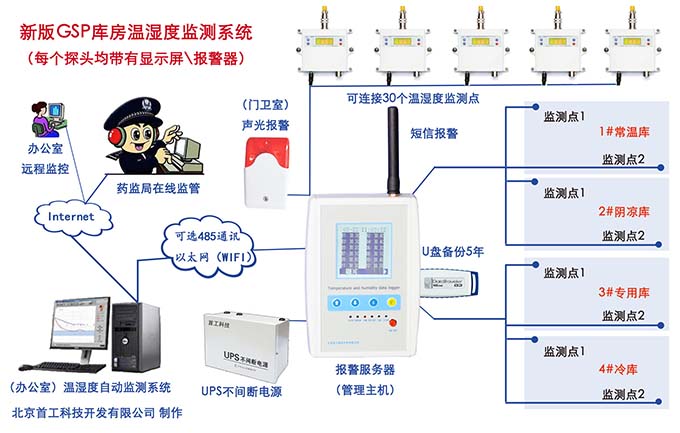
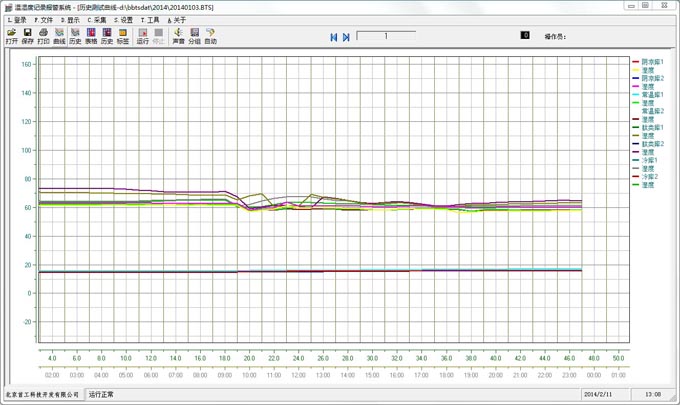
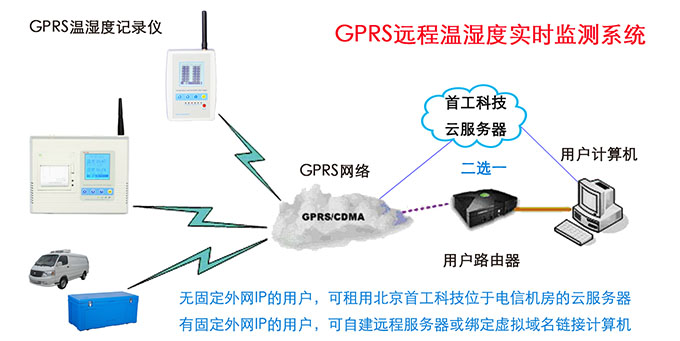
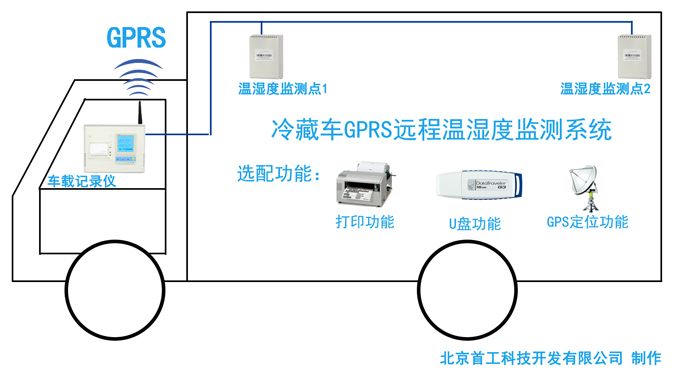
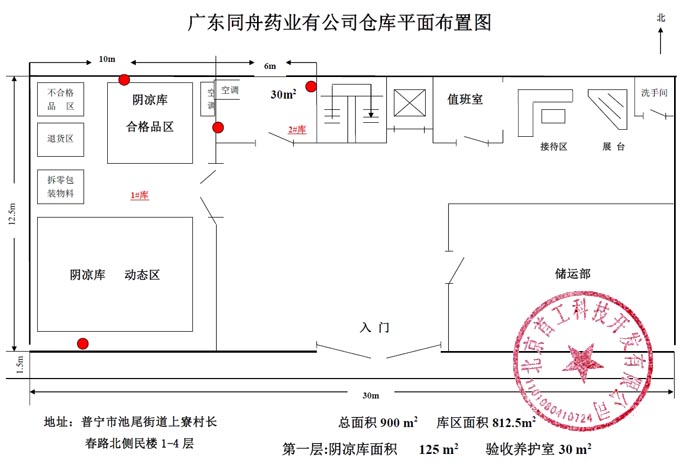
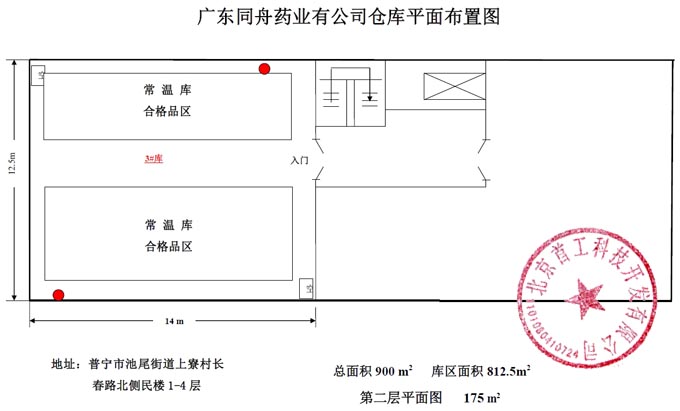
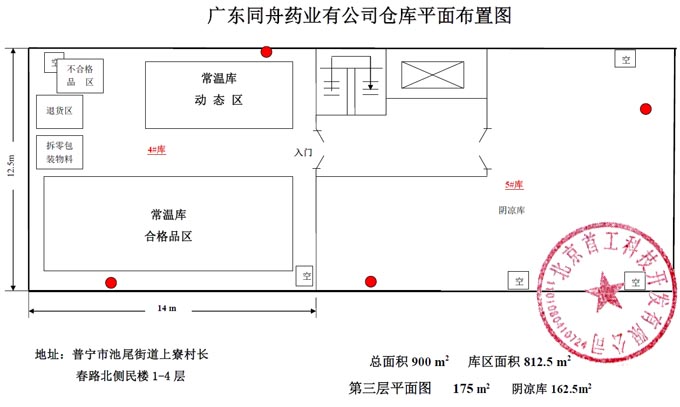
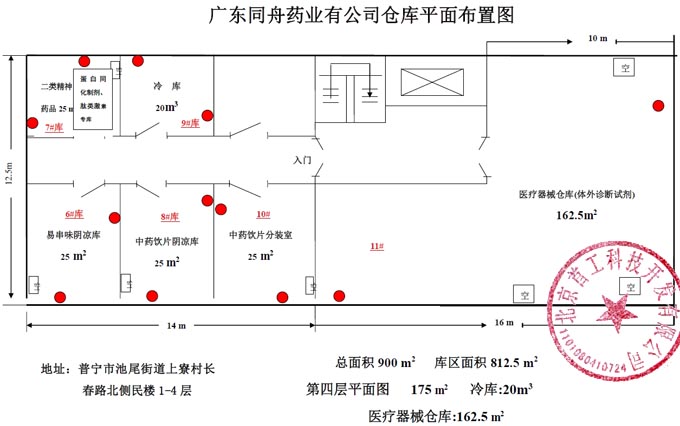
Tongzhou Pharmaceutical New GSP Temperature and Humidity Monitoring System Project Overview Project Name: Tongzhou Pharmaceutical New GSP Temperature and Humidity Monitoring System Construction unit: Guangdong Tongzhou Pharmaceutical Co., Ltd. Design Standard: "Drug quality management specification (Revised 2012)," Health Department Order No. 90 The new GSP Appendix 5: Appendix 1 "Storage and Transportation Management of Refrigerated and Frozen Drugs" Appendix 2 "Pharmaceutical Business Enterprise Computer System" Appendix 3, Automatic Temperature and Humidity Monitoring Appendix 4, Drug Receiving and Acceptance Appendix 5, "Verification Management" Chinese Pharmacopoeia (2010 edition) Project address: 1-4/F, Civil Building, Changchun Road, Shangyu Village, Chiwei Street, Puning City, Guangdong Province, China Engineering Contact: Manager Liu The project was approved by the Guangdong Province Food and Drug Administration GSP certification inspectors on site in December 2013 . I. Introduction to Temperature and Humidity Monitoring System Engineering Guangdong same boat Pharmaceutical Co., Ltd. established in 2000 is a private limited liability company. The company took the lead in Jieyang through the GSP quality certification. The main varieties sold Pioneer Pharmaceutical manufacturers are Shanghai, Shanghai New Asia Pharmaceutical Co., Shanghai New Asia Pharmaceutical Minhang Company , Shanghai Qingping Pharmaceutical , Shanghai Changcheng Pharmaceutical , Shanghai Sanitary Materials Factory , Shanghai Xinyi United Pharmaceutical , Zhejiang Zuoli Pharmaceutical , Zhejiang Xindonggang Pharmaceutical , Zhejiang Nanyang Pharmaceutical , Changzhou Kangpu Pharmaceutical , Jiangsu Four Ring Pharmaceutical , Jiangsu Peng Yao Pharmaceutical , Changzhou four drugs, etc. , the company's current agent species are : Shanghai New Asia 15g green ointment, Shanghai Xinya 10g Pikang Wang, Shanghai New Asia 0.64g sodium thiosulfate. June 2013, the same boat Guangdong Pharmaceutical Co., Ltd. Mr. Liu introduced by Shanghai Pharmaceutical Company, Division I find its design and installation of warehouse temperature and humidity monitoring system in line with the new GSP certification acceptance requirements. Based on the warehouse plan paper provided by the company and the requirements of the GSP appendix issued by the National Bureau , the company has formulated the construction plan based on the number of independent library rooms, the area of ​​the warehouse, and the number and distribution map of the monitoring points. The Treasury has two 20- cube pharmaceutical cold storage, easy-to-flavor library, second-class psycho-pharmaceutical repositories, and medical equipment (in-vitro diagnostic reagent library): 11 flat warehouses such as normal-temperature storage, shade storage, herbal medicine storage, and traditional Chinese medicine depot. The area of ​​warehouses is not less than 300 square meters. Each independent warehouse is installed with 2 road temperature and humidity monitoring points in accordance with the new GSP appendix . The system consists of a temperature and humidity alarm recorder host and 12- channel probe monitoring terminals, as well as temperature and humidity monitoring software. Second, the temperature and humidity monitoring system introduced: Warehouse temperature and humidity recorder +22 temperature and humidity monitoring point + temperature and humidity automatic monitoring system software The system was designed in strict accordance with the requirements of the automatic monitoring of temperature and humidity in the "Pharmaceutical Business Quality Management Regulations ( 2012 Revision) " Order No. 90 (new version of GSP ) and the appendix. The temperature and humidity recorders can be docked with the SFDA platforms across the country (the local Food and Drug Administration needs an open interface). The automatic temperature and humidity monitoring system consists of a measuring point terminal ( temperature and humidity sensor ) , a management host ( pharmacy warehouse temperature and humidity recorder ) , a data backup memory, a UPS uninterruptible power supply, and a temperature and humidity monitoring system software that comply with the new GSP requirements. Automatic monitoring and recording of humidity, SMS alarm, power off alarm, UPS uninterruptible power supply, data backup, automatic data upload and other functions, meet the on-line regulatory requirements of the Food and Drug Administration, the temperature and humidity monitoring system and the cooling system are set separately, independent operation, in line with the new version of GSP All acceptance requirements. The main function of the warehouse temperature and humidity automatic monitoring system : Automatic recording of temperature and humidity : data update interval: 1 minute / time, regular recording: 30 minutes / time, automatically changing to 2 minutes / time when the alarm occurs . Temperature and humidity alarm function : critical temperature and humidity limit alarm + power alarm Temperature and humidity alarm : local sound and light alarm + computer system sound alarm + SMS remote alarm ( 1-8 mobile phones) + telephone alarm UPS uninterruptible power supply : temperature and humidity recorder with uninterrupted power supply, ( UPS life is not less than 5 hours, power supply can increase capacity for 24 hours) Temperature and humidity data backup : backup data can be stored for more than 5 years , temperature and humidity recorders use U disk backup memory (maximum support 32G ) Temperature and humidity monitoring system alarm description: 1 , a repeater can be connected to 32 single-point display temperature and humidity recorders, warehouse temperature and humidity monitoring system, the total number of measuring points in 32 or less only requires a temperature and humidity alarm processor; 2 , external large-capacity UPS uninterruptible power supply, when the external power supply stops, automatically switch to the UPS power supply and send SMS alarm. 3 , the temperature and humidity exceed the upper and lower limits or when the power supply is interrupted, the local sound and light alarm is issued, and alarm messages are sent to 1-8 designated mobile phones. 4 , temperature and humidity recorder U disk backup memory (maximum support 32G ), backup data can be stored for more than 5 years Single temperature and humidity recorder (with display) : 1, (0.11.8%RH) 2,4 3,-55+125 4,0100% RH 5, 6, 7. 8, the effective transmission distance of the signal line can reach up to 1000 meters. Refrigerated car GPRS remote temperature and humidity monitoring system Third, the number of warehouse temperature and humidity monitoring points calculation and installation distribution requirements: GSP Appendix 3 Automatic Temperature and Humidity Monitoring Article 13 The number and location of measuring point terminals installed in drug warehouses or warehouses shall meet the following requirements : (A) for each separate drug cartridge mounting warehouse or terminals at least two measuring points, and evenly distributed. (Ii) in the plane of the storage area of 300 m2 or less, at least two measurement points mounted terminal; 300 square meters per increase by at least 300 square meters measuring a terminal point, less than 300 square meters of 300 square meter . Warehouse plane terminal mounting position of the measuring point, shelf drugs or drug not less than 2/3 of the height of the stack pallet. (Iii) automated warehouse rack warehouse or shelf storey between 4.5 to 8 m per 300 m2 area of the mounting terminals at least four measuring points, each additional increase of at least 300 square meters two measuring points terminal, And evenly distributed on the shelves, under the position; shelf height above 8 meters , at least 6 measuring point terminal installed per 300 square meters area , each additional 300 square meters at least 3 measuring point terminal , and evenly distributed in the shelf The upper, middle, and lower positions; less than 300 square meters are calculated based on 300 square meters. The location of the upper measuring point terminal of the elevated warehouse or fully automatic three-dimensional warehouse shall not be lower than the highest position of the uppermost shelf storage medicine. (4) The number of installations for storing the measuring point terminals of cold storage and frozen drug warehouses shall meet the requirements mentioned above in this section. The installation quantity shall be calculated for every 100 square meters . Article 14 The number of measuring point terminals installed in each independent refrigerated or frozen pharmaceutical transport vehicle or carriage shall not be less than two . For vehicles with a volume of more than 20 cubic meters, at least one terminal shall be added for each additional 20 cubic meters , and 20 cubic meters shall be calculated for less than 20 cubic meters. Each refrigerated box or incubator should have at least one measuring point terminal. Fourth, the temperature and humidity monitoring system verification report preparation (a), user demand program confirmation URS Tongzhou Pharmaceutical New GSP Temperature and Humidity Monitoring System User Requirements Program Verification URS Engineering name: the same boat Pharmaceutical The new GSP temperature and humidity monitoring system Project Location: Changchun Road, north of Guangdong Province Liaocun Man House on floors 1-4 Puning City, Jieyang City, Chi Mei Street Project content: 3 normal Reservoir, seven cool reservoir, a cold storage, refrigerated trucks 1, an incubator 5 Design requirements: Party B designs the automatic temperature and humidity monitoring system for drug storage and storage (warehouse and transport refrigeration equipment) of Party A according to the "Pharmaceutical Business Quality Management Regulations ( Revised in 2012 ) " and the appendix, and the temperature and humidity conditions of the drug storage process The temperature status of the frozen and frozen medicines during transportation is automatically monitored and recorded in real time, which effectively prevents risks that may occur during storage and transportation that affect the quality and safety of medicines, and ensures the quality and safety of medicines. According to the requirements of the specification, the automatic temperature and humidity monitoring system shall be verified to confirm that the related facilities, equipment and monitoring system can meet the specified design standards and requirements, and can be safely and effectively operated and used normally to ensure that the refrigerated and frozen drugs are stored and transported. Quality safety in the process. Project Requirements: Details of the project of installing automatic temperature and humidity monitoring system (see figure paper for details) Monitoring project Monitoring point details URS1: Warehouse Distribution warehouse: a layer # 1, # 2, Layer # 3, Layer # 4, # 5; Layer # 6, # 7, # 8, # 9, # 10, # 11 Warehouse type area height Number of measuring points 1# shading on the first floor: Internal Area: 125 m2 Drug shelf / stack height: 2 meters 2 2# first floor conservation library: Internal Area: 30 m2 Drug shelf / stack height: 2 meters 2 3# second floor room temperature library: Internal Area: 175 m2 Drug shelf / stack height: 2 meters 2 4# third floor room temperature library: Internal Area: 175 m2 Drug shelf / stack height: 2 meters 2 5# shading on the third floor: Internal Area: 162.5 m2 Drug shelf / stack height: 2 meters 2 6# is easy to smell on the fourth floor: Internal Area: 25 m2 Drug shelf / stack height: 2 meters 2 7# fourth floor second class library: Internal Area: 25 m2 Drug shelf / stack height: 2 meters 2 8# fourth floor medicine library: Internal Area: 25 m2 Drug shelf / stack height: 2 meters 2 9# fourth floor cold storage: Internal Area: 10 m2 Drug shelf / stack height: 2 meters 2 10# fourth floor of the library: Internal Area: 25 m2 Drug shelf / stack height: 2 meters 2 11# fourth floor reagent library: Internal Area: 162.5 m2 Drug shelf / stack height: 2 meters 2 URS2: Refrigerated car Refrigerated vehicle : 1 vehicle, volume: 5 m3 URS3: Incubator Incubator: 5, five incubator with temperature and humidity recorder Project Requirement Unit Guangdong Tongzhou Pharmaceutical Co., Ltd. confirmed: (Chapter) (II) Verification Report Temperature and humidity automatic monitoring system project Verification scheme Project Time: June 1, 2013 to November 10, 2013 Project Location: Changchun Road, north of Guangdong Province Liaocun Man House on floors 1-4 Puning City, Jieyang City, Chi Mei Street Project Name: Temperature and Humidity Automatic Monitoring System Project Validation Criteria: "Pharmaceutical Business Quality Management Regulations ( Revised in 2012) " and Appendices Verification reference: "Pharmaceutical GMP Guide" plant facilities and equipment - online monitoring system verification Verification steps: development of implementation plan - design and installation plan confirmation DQ - validation plan confirmation - equipment installation verification IQ - system operation verification OQ - system performance verification PQ (customer inspection process) - training - results overall evaluation - - Preparation of verification report - acceptance of delivery. Please consult the Beijing GSP expert for complete temperature and humidity monitoring system program : 010-51753122 , 13381084848 Beijing First Engineering Technology Development Co., Ltd. 2013.6.1 Fourth, check out the links: Guangdong pharmaceutical enterprises GSP certification publicity Bulletin No. 199 Http:// GSP Certification Public Notice Announcement No. 199 Release time: 2014-1-22 According to the "People's Republic of China Drug Administration Law" and its implementation regulations, the SIPO organizes certification inspections in accordance with the "Administrative Measures for the Administration of Pharmaceutical Business Quality Management Standards," and the following 96 pharmaceutical companies meet the requirements of the "Pharmaceutical Business Quality Management Regulations". It is now publicly announced and the community is required to supervise it. Supervision Tel: ( 020 ) 37886148 Address: No. 753 , Dongfeng East Road , Guangzhou City Postal Code: 510080 No. Company Name Certification scope (business model) Business Scope Business location Business Address On-site inspection time Site inspector 5 Guangdong Tongzhou Pharmaceutical Co., Ltd. wholesale Chinese herbal medicines ( acquisition ) , Chinese Herbal Medicines, Chinese patent medicines, chemical raw material medicines, chemical medicine preparations, antibiotics raw material medicines, antibiotic preparations, biochemical medicines, biological products ( including in vitro diagnostic reagents ; addition to vaccines ) , and second class psychoactive drugs ( preparations ) , anabolic agents, peptide hormones ** Jieyang City No. 104 , No.4 , Huacheng Garden Phase I, North of Jiangbei Road, West of Xiaocui Road, Dongshan, Jieyang City December 28, 2013 to December 29, 2013 Xu Muping, Chen Buxiang, Lin Biao Keywords: temperature and humidity recorder , temperature and humidity monitoring of the warehouse, automatic monitoring of temperature and humidity, verification of temperature and humidity monitoring system, GSP cold chain certification
Tungsten carbide roller has characteristics of good wear resistance, high temperature red hardness, thermal fatigue resistance and thermal conductivity and high strength , have been widely used in high-speed wire rod, bar, rebar, seamless steel tubes, etc. Domestic production of tungsten carbide roller materials mostly WC- Co, WC- Co- Ni- Cr two series, and the content of Co, Co- Ni - Cr is in the range of 6wt% ~ 30wt%. From the use of perspective, tungsten carbide rollers has good mechanical properties, its flexural strength up to 2200 MPa or more, shock toughness up (4 ~ 6) × 10^6 J/ m^2, Rockwell hardness (HRA) is up to 78 to 90, widely in the high-speed wire rod rolling process, which is much higher than single-slot chilled cast steel or high speed steel rolls.Tungsten carbide is made of Tungsten Carbide Powder and binder phase (such as drilling, nickel, etc.), and then pressing and sintering, regardless of the conditions under cold rolled or hot rolled has excellent wear resistance, tungsten carbide rollers has been widely used in pre-finishing mill and finishing of high-speed wire rod currently. On the performance of tungsten carbide roller in hot-rolling wire rod , the material must meet the following requirements:
Tungsten carbide roller material design
Tungsten carbide roller category
Tungsten carbide roller production process control Carbide Roller,Carbide Ring,Mill of Rolling Line,Steel Wire Rod,Mill Rolling Finish Stand,Mill Roller Groove Type Luoyang Golden Egret Geotools Co., Ltd , https://www.xtchvaf.com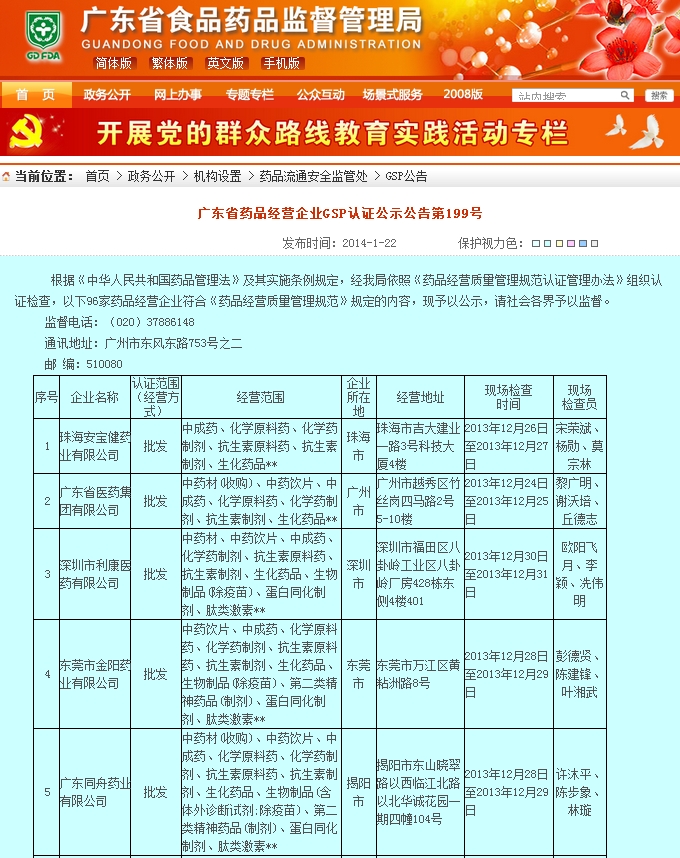




1. Sufficient anti-fracture strength;
2. Good abrasion resistance;
3. The smooth surface finish;
4. Excellent corrosion resistance, thermal fatigue, thermal cracking performance.
Tungsten carbide roller rings can working in bad conditions , small profile rolling (especially rebar rolling) process conditions is harsher than the high-speed wire rod, and therefore corresponds to the profile rolling ,tungsten carbide rollers recommend using high binder phase carbide.
Pre-finishing all vehicles roller should ensure its high toughness, strength, rigidity and thermal conductivity, followed before considering its wear resistance. When designing each vehicles roller, pre-finishing materials should choose carbide grades of Co, Co- Ni- Cr binder content is high (greater than or equal to 25wt%) , requiring an average WC grain size of coarse (5μm ~ 6μm), to obtain a higher shock toughness, proper strength and hardness. For the finishing of the roller movements, particularly the last two rollers of the finish rolling, which suffered load is small, and high relative velocity of the material to be pressed (80 m / min ~ 120 m / min). In this case, the wear resistance of the roller to be the most important requirements, and must ensure the strength , timpact toughness and hardness of a reasonable match, so the binder of Co / Ni content ratio and the average grain WC control of particle size and other factors must have greater control in front of different pre-finishing rolling roll.
According to the structure of tungsten carbide rollers, it can be divided into solid tungsten carbide roller and composite tungsten carbide roller. Solid tungsten carbide rollers have been widely used in pre-finishing and finishing stands high speed wire rod mill (including fixed reducing the rack, pinch roller rack). Composite tungsten carbide roller is made of cemented carbide and other materials, and it can be divided into tungsten carbide composite roll rings and solid tungsten carbide composite roller. Tungsten carbide composite roll rings mounted on the roller shaft; solid tungsten carbide composite roller will be directly cast in the roll axis to form a whole, a large load is applied to the rolling mill.
Tungsten carbide rollers produced by powder metallurgy method, the key to its process control is the chemical composition of the material and the mixture was prepared, pressed molding, sintering and deep processing and other preparation process parameters.
1. Preparation of starting material (WC focus quality): As the WC raw material and quality control of the use of different levels of quality may fluctuate, resulting in adverse effects on microstructure.
2. Preparation of the mixture: Mixture preparation is the key to the production process of the roller, the roller of failure modes - trachoma, mainly generated by this procedure.
3. Pressing: roller pressing is an important process of the roller mill.
4. Sintering: roller sintering is to determine the final quality of the roller production processes, use of advanced low-pressure sintering technology, HIP sintering technology can greatly improve the performance of roller.
5. Deep processing: deep processing rollers have a greater impact on the quality and accuracy of the roller surface.
March 19, 2024