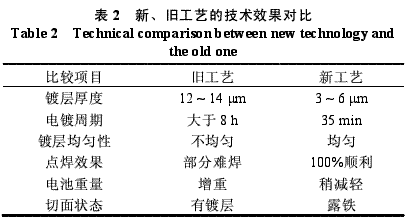
1 Introduction The tabs (conductive sheets) are thin-cut parts of a 0.2 mm thick steel strip (see Figure 1) and are important components for high-rate battery conduction. Plating of sheet parts is very difficult, but in order to ensure the performance of the product, the finished shop is easy to process, and the quality of plating must be improved. Since the opening high-rate battery products have been put into production, the conductive sheets have been using the nickel-plated nickel plating process, which has many disadvantages, such as many waste products, many repaired products, difficult selection, long plating cycle, low output, etc. The quality is not good enough to meet the production needs. . It takes 8~10h to roll a barrel of conductive sheet, 2h for drying, 2h for the selection, and the thickness of the coating is uneven. Nickel enamel often appears on the thick side, and the thin side often leaks and rusts. The pass rate is generally 70. %the following. After going to the next process, quality feedback is often caused by the difficulty of spot welding or partial rust due to the partial thickness of the nickel layer. Later, the method of hanging plating was adopted, but the conductive sheet was always thick at the upper end and thin at the upper end. It was generally difficult to spot weld the finished product shop, and the problem of burning the spot welder coil often occurred. In summary, the electroplating method and process of the conductive sheet must be improved to meet the quality requirements. In order to change the above situation, a process of first plating a 0.2 mm × 37 mm steel strip and then punching it into a conductive sheet is proposed. At first, some people thought that it would not work. The reason was that the cut surface was easy to rust during the inventory period and in the cleaning process of the finished shop. But the author believes that rusting during inventory and cleaning can be solved. Therefore, rust prevention is the main target, and the exploratory process test is first carried out, and then the batch test from small to large is carried out until the production is started. 2. Process analysis and experimental verification 2.1 Exploratory process test situation A 0.2 mm × 37 mm steel strip was electroplated with nickel 6 μm, and 1 kg was punched into a conductive sheet. Dip different rust preventive solutions separately, and leave them for a period of time to observe the rust of the cut surface. The formulation and operating conditions of different anti-rust solutions are as follows. (1) General engine oil, left to dry after dipping. (2) Anti-rust water: 200g/L sodium nitrite + 150g/L urea, dissolved in distilled water, room temperature, immersed and left to dry. (3) Canal brand 8211 anti-rust cleaning agent: tap water dissolution, mass fraction 5%~8%, room temperature, drying after drying. (4) Anti-rust alkali solution: 5g / L sodium hydroxide + 5g / L sodium carbonate, dissolved in tap water, room temperature, dipped and dried. (5) Directly dry and store after slicing. The test results are shown in Table 1. It can be seen from Table 1 that during storage, the anti-rust effect of dip oil or immersion rust water is the best, followed by cleaning agent and lye. This is the result of the first phase of the test. The second phase focuses on solving the problem of no rust during the cleaning, dehydration and drying of the finished product workshop. According to the original cleaning process (washing-alcohol dehydration-drying-spot welding), about 50% of the finished products are yellowish (rusty). It can be seen that the original cleaning process in the finished product shop does not work and must be improved. Since iron and nickel are very stable in an alkaline environment, they are immersed in a dilute alkali solution and dried without causing chemical corrosion. In addition, although the potential of iron is higher than that of nickel, the electrochemical corrosion of iron can be overcome because sodium hydroxide has an effective corrosion inhibition effect on the anode process of iron in electrochemical corrosion, and the cathode process of sodium carbonate on nickel. It also has an effective inhibitory effect. Therefore, the nickel-plated cut surface of the iron does not cause significant corrosion of iron in the medium of sodium hydroxide and sodium carbonate. After trial and production practice, a reasonable cleaning process was explored. 2.2 New process and operation requirements The process is: 0.2mm × 37mm steel strip electroplated nickel 6μm - die-cut into conductive sheet - dipping anti-rust water storage - hot water wash - cleaning agent degreasing - hot water washing - cold water washing - dip solubilized (mass fraction 2% carbonic acid Sodium or Sodium Hydroxide) - Alcohol Dehydration - Drying - Dispersing Drying or Rolling Drying - Spot Welding. The process requirements are as follows: (1) The 0.2mm × 37mm steel strip electroplated nickel layer must be dense, the bonding force is firm, and there must be no insufficient brightness or loss of light. (2) The warehouse should be mainly based on the storage of electroplating belts. The production plan should be arranged as much as possible. The plating should be shortened as much as possible. In special cases, it should be immersed in rust-proof water. (3) The punching process should use as little oil as possible to prevent the next process from being oily and cleaned with water. (4) The cleaning process of the finished product workshop must strictly achieve a soaking alkali concentration of 2% and fluctuations from 1% to 3%. When the concentration of the lye is low, the cut surface is yellow and it is difficult to spot weld. (5) It is strictly forbidden to accumulate too thick during the drying process after dehydration, otherwise it is not conducive to water dispersion. It should be spread as thin as possible and flipped regularly. It should be noted that the conductive sheets after punching are strictly prohibited from high temperature and high humidity in parallel, and can be dip solubilized or rust-proof water in summer. 2.3 Experimental verification It has been verified by successive batch production of 30, 100 and 200kg, and the effect is good. The finished product workshop can be cleaned according to the new process, and can achieve 100% cut surface without yellowing, uniform spot welding, stable quality, and breakthrough in quality and output. 3. Technical and economic effects 3.1 Comparison of technical effects between new and old processes The technical effects of the new and old processes are shown in Table 2. As can be seen from Table 2, in the first five comparisons, the new process is clearly superior to the old process. Only the last item, the cut surface, is the problem to be solved in this work. The production application proves that the new process is not only feasible, but also facilitates the operation of the finished product shop. There is no problem of spot welding, and no nickel plaque and large area rust are found. The new process has a short production cycle, large output, and uniform quality. 3.2 Economic effects (1) Improve work efficiency. The original barrel plating process has a long production cycle and uneven plating; while the 0.2mm×37mm steel strip has a short production cycle and uniform plating. It is estimated that the plating belt is 3.8 times of the barrel plating output, which means that the new process has a net increase of 2.8 times, and there is very little waste. According to the annual output of 137t, the plating time requires 22,459 people per hour, while the barrel plating requires 85,625 hours per person, saving 63,166 people per hour and saving labor costs of 3,5530.8 yuan. (2) Reduce the scrap rate. The rejection rate of barrel plating is generally around 30%, while the rejection rate of plating belt is generally 0.3% to 1.5%. (3) Saving metal nickel. The thickness of the nickel plating of the conductive sheet is 12 to 14 μm, and the thickness of the nickel plating is 3 to 6 μm. Calculated according to 9213.7t, the annual saving of nickel 519.35kg, calculated according to the nickel price of 60 yuan / kg, a total savings of 31161.27 yuan. Commercial
grade perforated cookie sheets made of durable 19 Gauge aluminum, these
professional-weight Baking Sheet pan features a wire reinforced beaded
rim and is heavier in gauge than that of standard home bakeware. Compared with low
quality Sheet Pan, this commercial grade sheets make sure duality and strength
and without worry of it bending or warping under the weight due to reinforced
rim provides a secure grip for easier transport. Size of this cookie sheet will
be customized by clients. This pan's tapered design allows for simple
stacking to save valuable storage space in your commercial bakery or restaurant
kitchen. Full Perforated Heavy Bun Pan,Perforated Baking Tray,Aluminum Alloy Full Sheet Pan,Aluminum Cooking Sheet Pan YANGZHOU GNEWY MACHINERY EQUIPMENT CO.,LTD , https://www.gnewy.com

June 04, 2021